The results of the bidding in China's fourth-round national centralized drug procurement have been announced recently. Four drugs of Jiangsu Hansoh Pharmaceutical Group Co., Ltd. ("Hansoh Pharma" or "Company") are among the winners, specifically: Fulaidi (repaglinide tablets), Fulairui (canagliflozin tablets), Fulaixin (empagliflozin tablets) and Xintai (bortezomib for injection). Hansoh Pharma's inclusion in this national centralized drug procurement once again for the four drugs embodies its product quality and comprehensive strength, and also demonstrates its scientific research and innovation capability. Since the introduction of centralized drug procurement across "4+7" cities, Hansoh Pharma has actively responded to the call of the national policy, and has participated in and won the bid in each round of centralized procurement. Through its practical actions in technological innovation and lean manufacturing as well as its unremitting efforts in continuously improving the accessibility of drugs for patients, the Company has brought benefits to patients, made contributions to communities, and established positive and good social influence.
Fulaidi (repaglinide tablets)
Fulaidi (repaglinide tablets) is a key variety of Hansoh Pharma's diabetes product line, indicated for patients with type II diabetes (non-insulin-dependent) whose hyperglycemia cannot be effectively controlled by diet, weight reduction and exercise.

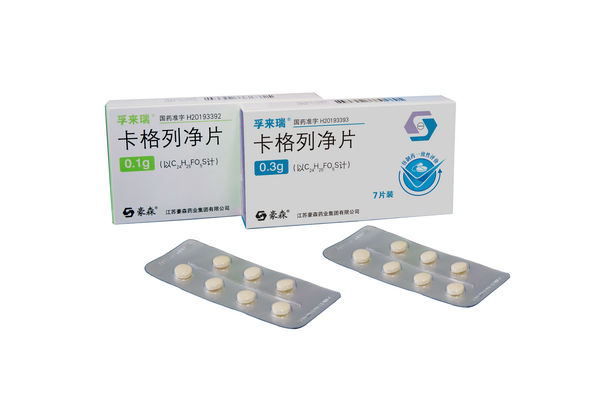
Fulairui (canagliflozin tablets)
Fulairui (canagliflozin tablets) was approved for marketing in December 2019 as an SGLT-2 inhibitor for patients with type II diabetes.
Fulaixin (empagliflozin tablets)
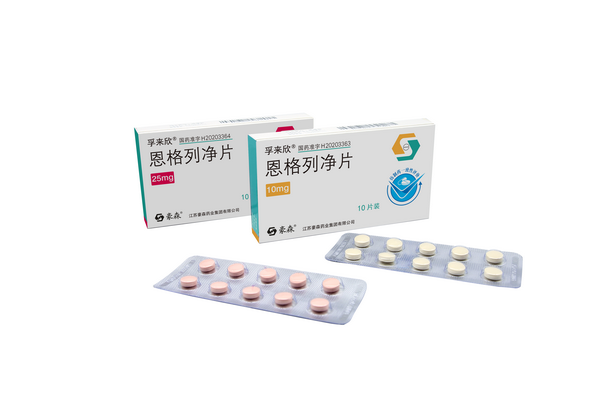
In July 2020, Fulaixin (empagliflozin tablets) was approved for marketing and also deemed to have passed the consistency evaluation. Fulaixin is a highly selective sodium-glucose cotransporter 2 (SGLT-2) inhibitor that, in addition to its clear glucose-lowering effect, reduces body weight, the risk of cardiovascular events and the progression of nephropathy in patients with diabetes.
Xintai (bortezomib for injection)
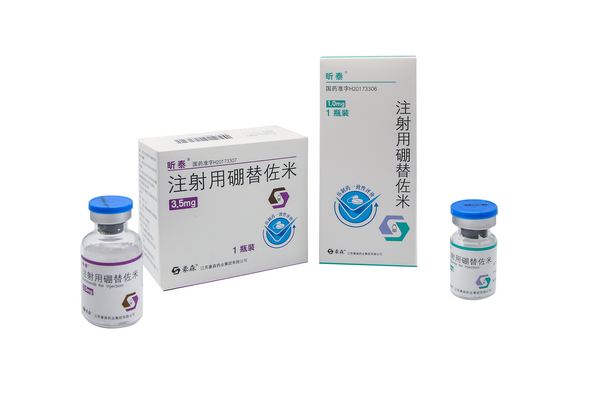
Xintai (bortezomib for injection), a proteasome complex inhibitor for the treatment of multiple myeloma and mantle cell lymphoma, was approved for marketing in November 2017, bringing benefits to patients with multiple myeloma and mantle cell lymphoma in China.
The inclusion of the four drugs in this round of centralized procurement is expected to reduce the drug burden of patients in the fields of oncology and diabetes, improve the accessibility of drugs, help improve the standardized treatment level, and bring actual benefits to patients. Upholding the corporate mission to "create excellence in pharmaceuticals, enhance innovation in China", Hansoh Pharma will in the future accelerate technological innovation, introduce more quality drugs that are affordable and accessible to patients, and contribute and dedicate to the development of China's pharmaceutical industry and the improvement of national health.。
