Recently, the clinical trial application of Jiangsu Hansoh Pharmaceutical Group Co., Ltd. ("Hansoh Pharma" or the "Company") for HS-20094, an independently developed innovative drug, was accepted by the National Medical Products Administration (NMPA).
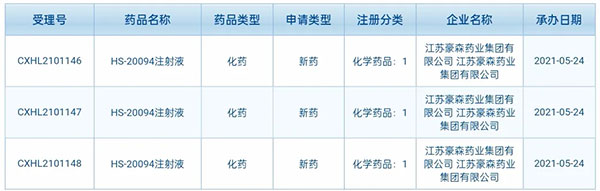
▲ Screenshot of public information from CDE, NMPA
HS-20094 is a new-generation GIP/GLP-1 dual agonist independently developed by Hansoh Pharma, intended for the treatment of diabetes.
