On March 1, 2023, the National Reimbursement Drug List for Basic Medical Insurance, Work-Related Injury Insurance and Maternity Insurance (2022) was officially implemented nationwide. Hansoh Pharma's Ameile (Almonertinib Mesylate Tablets), China's first original third-generation EGFR-TKI for first-line treatment, was prescribed as a reimbursable drug for the first time in a number of hospitals and grassroots medical institution across China.
On the morning of March 1, Chen Dan, Chief Physician of Department of Respiratory Medicine, Leshan People's Hospital in Sichuan, issued the first prescription of Ameile in the hospital. According to the requirements of the National Healthcare Security Administration for the supply guarantee of negotiated drugs, Ameile has been rapidly included in the "dual-channel management" scope across China, and can be purchased as a reimbursable drug at designated pharmacies.
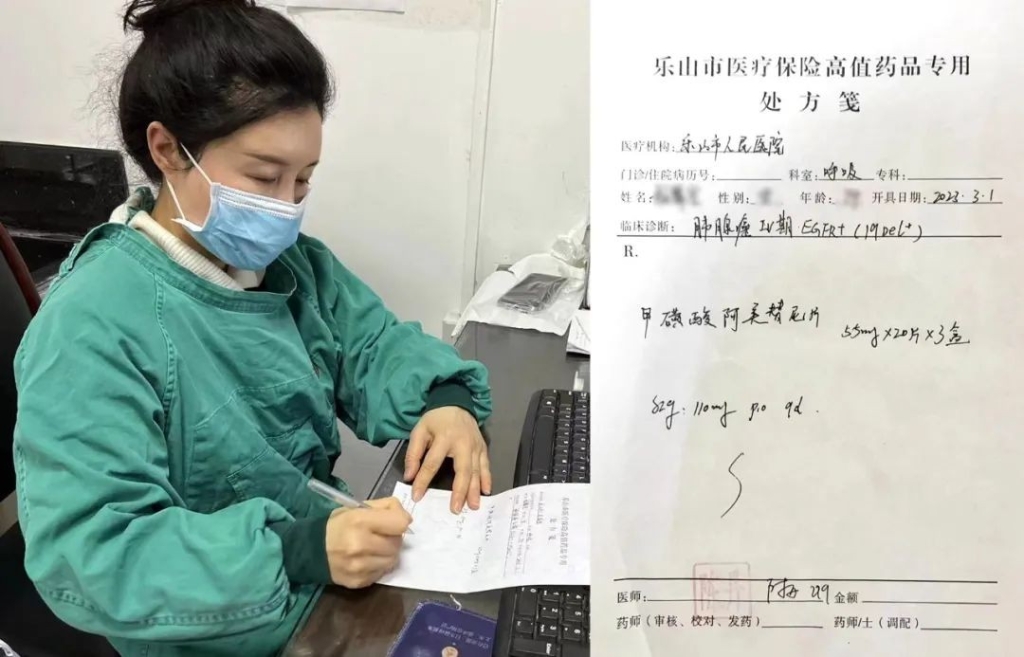
Physician Chen Dan prescribed Almonertinib Mesylate Tablets as a reimbursable drug for first-line treatment
Ameile is a Class 1 innovative drug developed by Hansoh Pharma, and also China's first original third-generation EGFR-TKI. In March 2020, the first indication of Ameile was approved for marketing, filling the gap of third-generation EGFR-TKI in China; in December 2021, Ameile was approved as first-line therapy for adult patients with EGFR exon 19 deletion or exon 21 (L858R) substitution mutation-positive locally advanced or metastatic NSCLC; on January 18, 2023, Ameile was included in the new National Reimbursement Drug List for the benefit of more lung cancer patients.
Since its approval for marketing, Ameile has rapidly entered medical institutions and pharmacies at all levels nationwide and has been widely used in clinical practice, benefiting more than 200,000 NSCLC patients and winning unanimous praise from doctors and patients for its excellent efficacy and safety. On the first implementation day of the new National Reimbursement Drug List, Ameile was prescribed as a reimbursable drug for first-line treatment in a number of hospitals across China.

Jiangxi Cancer Hospital
To explore the therapeutic potential of Ameile in the lung cancer segment, Hansoh Pharma is also conducting multiple registered clinical studies, including multiple clinical studies of Ameile in combination with platinum-containing dual-agent chemotherapy for the first-line treatment of NSCLC with sensitive mutations and the adjuvant treatment of NSCLC, which will provide more NSCLC patients with a full range of multidimensional dosing options from early to late stage, from perioperative adjuvant treatment, first-line and second-line treatment to posterior-line treatment and bring more hope to lung cancer patients.
