The IDF Virtual Congress 2023 will be held from December 4 to 7. Phase 1 study data from Hansoh Pharma’s HS-20094 (a novel dual GIP and GLP-1 receptor agonist) will be released at the Congress in the form of posters and oral presentations. This study shows that HS-20094 exhibites good safety, well tolerance, and a good effect in reducing glucose and body weight in healthy adults.
Study Summary
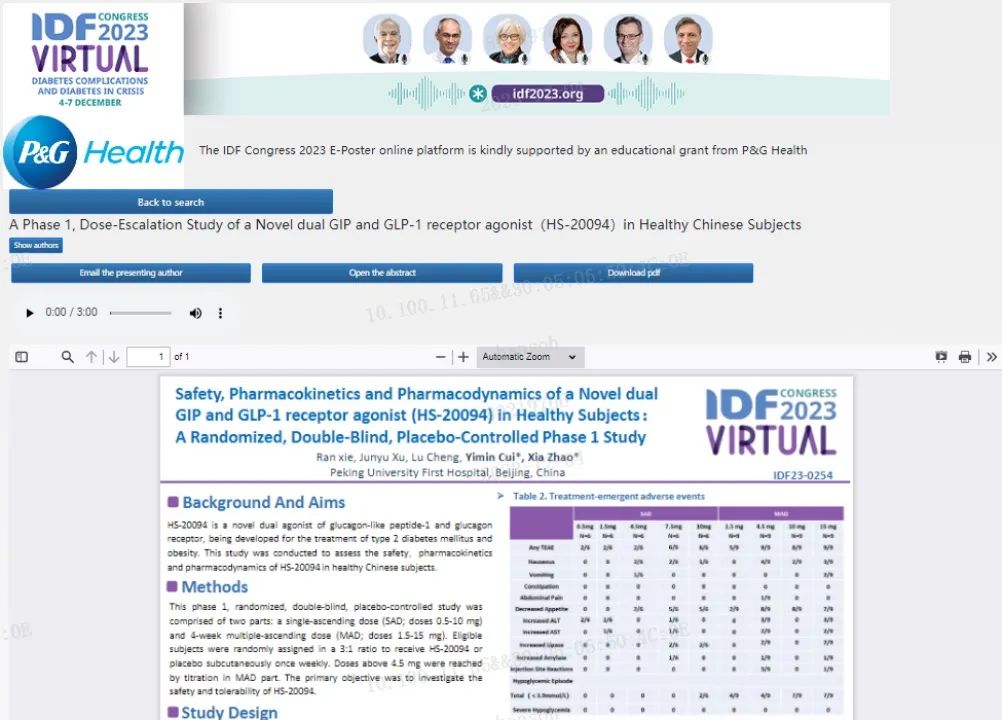
[Study title] A phase 1 study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of HS-20094 administered by single and multiple subcutaneous injections in healthy subjects
[Abstract No.] IDF23-0254
[Study methods] This randomized, double-blind, placebo-controlled dose-escalation study consisted of two stages: a single-ascending dose (SAD; doses 0.5-10 mg) phase and multiple-ascending dose (MAD; doses 1.5-15 mg) phase
[Study results]
Safety: Overall, HS-20094 was safe and well tolerated. The most frequent side effects reported with HS-20094 were decreased appetite and nausea, both of which were considered to be mild to moderate in severity. No increase in dose-dependent gastrointestinal adverse events was observed.
Pharmacokinetics: Following a single dose of HS-20094 0.5-10 mg, the median Tmax ranged from 8 to 48 hours, and the mean t1/2 was 160 to 166 hours; after 4 consecutive doses of HS-20094 1.5-15 mg, the mean of T1/2 was 155.8 to 169.9 hours.
Pharmacodynamics: During the MAD phase, after subjects received once-weekly administration of HS-20094 via subcutaneous injection for four consecutive doses, AUC0-2h glucose on day 22 of HS-20094 group was lower than that in placebo group, and there was no significant difference between the two groups in AUC0-2h insulin on day 22. A dose-dependent weight loss was observed in the MAD part. The mean (SD) reduction of body weight from baseline was 4.74 (1.687) kg on day 29 in 15mg dose cohort compared to 0.45 (1.460) kg in the placebo cohort. The efficacy of weight loss maintained for at least 4weeks after stopping the drug.
According to the IDF report, approximately 643 million adults (aged 20-79 years) worldwide are expected to be diagnosed with diabetes by 2030, and may rise to 783 million in 2045. During this period, the world population is estimated to grow by 20%, while the number of diabetic patients is estimated to increase by 46% [1]. The prevalence of type 2 diabetes in China also shows an increasing trend year by year. The rate climbed up to 11.2% from 2015 to 2017, with type 2 diabetes accounting for over 90% of the total population [2], demonstrating a significant unmet clinical need.
Glucagon-like peptide-1 receptors (GLP-1Rs) are a new type of hypoglycemic drugs that enhance insulin secretion and inhibit glucagon secretion in a glucose concentration-dependent manner. Activation of GLP-1Rs can delay gastric emptying and reduce food intake through appetite suppression as they act on the central nervous system, resulting in a blood glucose-lowering effect. In addition to their proven glucose-lowering effects, these drugs can also reduce body weight, lower blood pressure, and protect the cardiovascular and renal organs [3,4]. Independently developed by Hansoh Pharma, HS-20094 is a dual GIP and GLP-1 receptor agonist that agonizes the downstream pathway by selectively activating both the GLP-1 and GIP receptors to realize biological effects such as reducing glucoseand body weight. The drug is administered once a week via subcutaneous injection. At present, Hansoh Pharma has initiated and accelerated a further study of HS-20094 in patients with type 2 diabetes, and overweight/obesity in China. It is hoped that the drug will bring benefits to these patients.
The International Diabetes Federation (IDF) is made up of more than 240 diabetes association members in 170 countries and territories, acting as the global voice of the diabetes community. It offers a global platform for discussion a wide range of diabetes issues from the latest scientific advances to education, in an effort to promote diabetes care, prevention, and treatment worldwide. The IDF Congress is the key annual meeting of the organization.
