Recently, the 3rd Hansoh Pharma Anti-Infection Summit Forum sponsored by Jiangsu Hansoh Pharmaceutical Group Co., Ltd. ("Hansoh Pharma" or "Company") was successfully held in Nanjing. Twenty academic opinion leaders from the three major fields of microbiology, surgery and intensive care medicine attended the forum to discuss the scientific diagnosis and treatment of infectious diseases and the standardized application of anti-infective drugs, which attracted the participation of more than 200 experts and clinicians from all over the country.

The 3rd Hansoh Pharma Anti-Infection Summit Forum was held in Nanjing
During the forum, Professor Wu Xiuwen, Deputy Chief Physician of General Surgery of the General Hospital of Eastern Theater Command, PLA, interpreted the newly released Guidelines for Diagnosis and Treatment of Abdominal Infection, the first of its kind in China. It is noteworthy that a large number of clinical studies have confirmed that Mailingda (Morinidazole and Sodium Chloride Injection) has reliable efficacy and good safety, and has been officially included in the Guidelines as one of the key recommended drugs for the treatment of abdominal infection.
Professor Wu Xiuwen interpreted China's first Guidelines for Diagnosis and Treatment of Abdominal Infection
Mailingda is a new-generation nitroimidazole anti-anaerobic innovative drug developed by Hansoh Pharma. It is the world's first nitroimidazole anti-anaerobic innovative drug in 40 years, with better efficacy and higher safety. In the surgical section of the forum, Professor Zheng Tao of the Research Institute of General Surgery of the General Hospital of Eastern Theater Command, PLA released the interim report on the prospective study of morpholinnitzole in preventing postoperative SSI. The latest research results show that the combination of Mailingda and broad-spectrum antibiotics can significantly reduce the incidence of SSI after abdominal infection by nearly 60% compared with the use of broad-spectrum antibiotics alone.
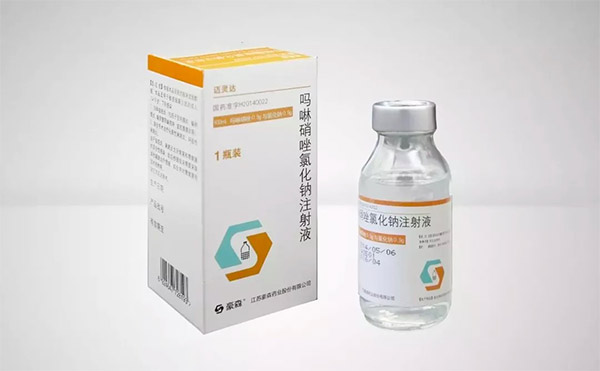
Innovative drug Mailingda (Morinidazole and Sodium Chloride Injection)
Hansoh Pharma is always focused on drug research and development in antibiotic resistance, an important field of public health. In addition to the innovative drug Mailingda, HS-10234, an anti-HBV (hepatitis B virus) Class 1 innovative drug for chronic hepatitis B, has entered clinical phase III. It is lower in dosage, more efficient and less toxic than existing drugs, and is expected to significantly reduce the risks of nephrotoxicity and osteoporosis of patients taking hepatitis B drugs. The Company has also launched a series of critical care anti-infection drugs such as Zetan Tigecycline for Injection), Hengsen (Micafungin Sodium for Injection) and Hengjie (Linezolid Glucose Injection), which fully cover the common clinical anti-infection fields.
The layout of these critical care anti-infection pipelines reflects the "clinical needs-centered" R&D philosophy of Hansoh Pharma. As an innovation-driven pharmaceutical company in China, Hansoh Pharma will continue its focus on the unmet clinical needs and strive to improve human health, create excellence in pharmaceuticals and enhance innovation in China through continuous innovation.
