The 115th Annual Meeting of the American Association for Cancer Research (AACR) was convened in San Diego from April 5 to 10. At this year's meeting, Hansoh Pharma released the results of the Phase I Study of HS-10370 monotherapy in a poster presentation. The study drug was a potent highly-selective small molecular KRAS G12C inhibitor (research and development code: HS-10370) administered orally, that was independently developed by Hansoh Pharma for the treatment of advanced solid tumors.
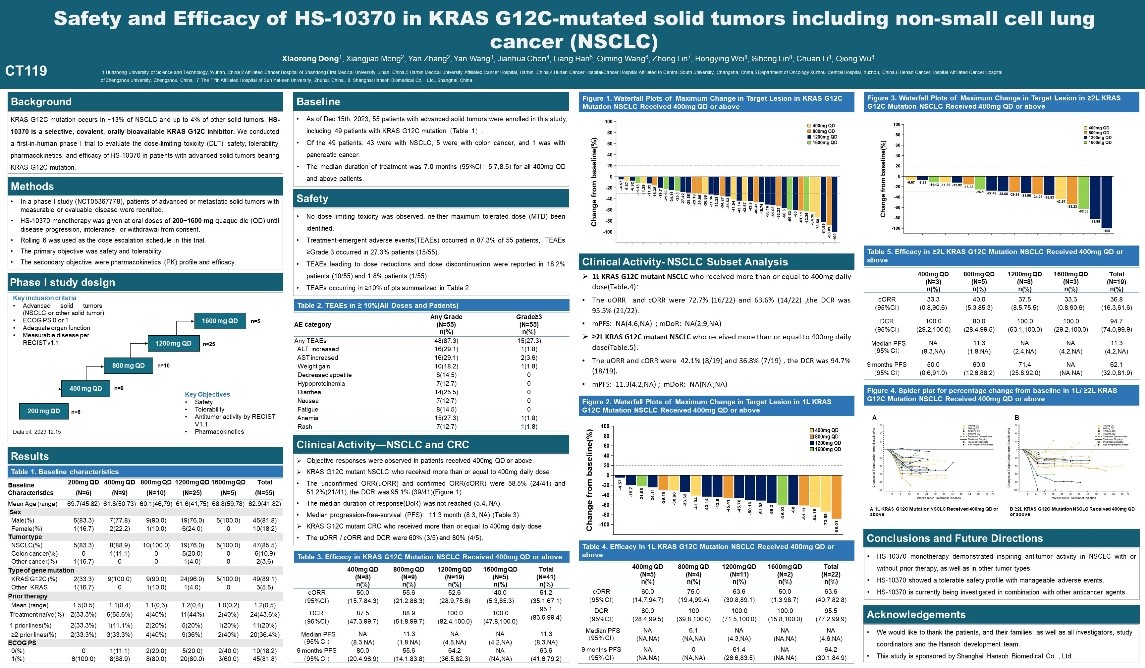
The results of the Phase I dose-escalation study show that HS-10370 demonstrates a favorable safety and tolerability profile in patients with advanced solid tumors; and the investigational drug also proves to have good efficacy in the treatment of advanced solid tumors (especially NSCLC) with KRAS G12C mutations. It is expected to become a new treatment option for cancer patients.
KRAS mutations cause proteins to grow uncontrollably and continuously activate downstream signaling pathways, thereby leading to cancer growth. Globally, KRAS G12C is known as the most common KRAS mutation subtype in lung cancer. However, no drug for this has yet been approved in China, and there is a significant unmet clinical need in this therapeutic area.
Hansoh Pharma will continue to explore the therapeutic potential of HS-10370 monotherapy and combination therapy in multiple tumor types, and expedite the approval and implementation of relevant clinical trials with the hope of providing patients with better regimes soon.
